
科研论文
Research and Articles
Liang Gong, Hongbao Sun, Li-fan Deng, Xia Zhang, Jie Liu, Shengyong Yang, and Dawen Niu*.
Journal of the American Chemical Society, 2019, 141, 19, 7680-7686
We demonstrate that readily available and bench-stable α-oxo-vinylsulfones are competent electro- philes in Ni-catalyzed Suzuki−Miyaura cross-coupling reactions. The C−sulfone bond in the α-oxo-vinylsulfone motif is cleaved chemoselectively in these reactions, furnishing C-aryl glycals or acyclic vinyl ethers in high yields. These reactions proceed under mild conditions and tolerate a remarkable scope of heterocycles and functional groups. Preliminary mechanistic studies revealed the importance of an α-heteroatom in facilitating these transformations.

Yingwei Wang, Li-Fan Deng, Xia Zhang,* and Dawen Niu*
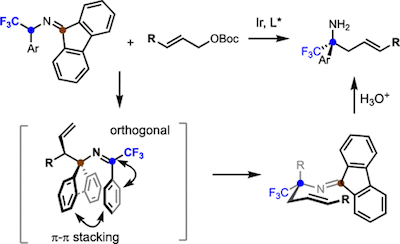
A general and mild method to prepare enantioenriched α-trifluoromethyl, α-stereogenic homoallylic amines is established. This reaction, which involves an Ir-catalyzed umpolung allylation of imines and a 2-aza-Cope rearrangement cascade, could yield both tetrasubstituted and trisubstituted stereocenters. This transformation employs readily available starting materials and displays broad substrate scope. The isolation and structural determination of reaction intermediates revealed factors critical for the efficiency and stereoselectivity of this transformation.
2018
Shaojian Tang,+ Xia Zhang,+ Jiayue Sun, Dawen Niu,* and Jason J. Chruma*.
Chem. Rev. DOI: 10.1021/acs.chemrev.8b00349
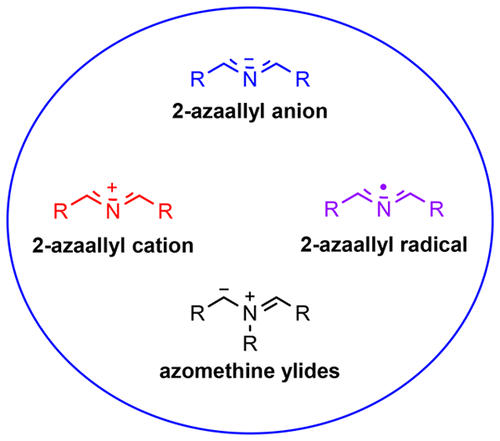
This review covers the use of 2-azaallyl anions, 2-azaallyl cations, and 2- azaallyl radicals in organic synthesis up through June 2018. Particular attention is paid to both foundational studies and recent advances over the past decade involving semistabilized and nonstabilized 2-azaallyl anions as key intermediates in various carbon−carbon and carbon−heteroatom bond-forming processes. Both transitionmetal-catalyzed and transition-metal-free transformations are covered. Azomethine ylides, which have received significant attention elsewhere, are discussed briefly with the primary focus on critical comparisons with 2-azaallyl anions in regard to generation and use.
Zhengyan Fu, Ning Deng, Sheng-Nan Su, Hongyang Li, Ren-Zhe Li, Xia Zhang, Jie Liu, and Dawen Niu*.
Angewandte Chemie International Edition. https://doi.org/10.1002/anie.201809391
We have achieved the asymmetric propargylic substitution (APS) reaction with 5H‐thiazol‐4‐ones by Cu/Zn dual metal catalytic system and the APS reaction with 5H‐oxazol‐4‐ones by Cu/Ti catalysis. These reactions furnish functional group‐rich, terminal alkyne‐containing products with two vicinal stereocenters in high yields and with good to excellent diastereo‐ and enantioselectivities. This study implies the use of dual metal catalytic systems as a viable approach to improve the selectivity profiles of the Cu‐catalyzed APS reactions.

Hong‐Bao Sun, Liang Gong, Yu‐Biao Tian, Jin‐Gui Wu, Xia Zhang, Jie Liu, Zhengyan Fu, and Dawen Niu*.
Angew. Chem. Int. Ed. 2018, 57, 9456 –9460
We have found that readily available N-alkyl hydroxylamines are effective reagents for the amination of organoboronic acids in the presence of trichloroacetonitrile. This amination reaction proceeds rapidly at room temperature and in the absence of added metal or base, it tolerates a remarkable range of functional groups, and it can be used in the late-stage assembly of two complex units.

Weidong Shang, Ze-Dong Mou, Hua Tang, Xia Zhang, Jie Liu, Zhengyan Fu, and Dawen Niu*.
Angewandte Chemie International Edition, 2018, 130(1), 320-324
Direct and site-selective O-arylation of carbohy- drates has been a challenge in synthesis. Herein we report a method based on copper-catalyzed O-arylation to address this challenge. Proper choice of the ancillary ligand on copper is critical for the efficiency and site selectivity of this trans- formation. This method features mild conditions, tolerates various functional groups, and demonstrates broad substrate scope.

2017
Ren-Zhe Li, Hua Tang, Liqiang Wan, Xia Zhang, Zhengyan Fu, Jie Liu, Shengyong Yang, Da Jia, and Dawen Niu*.
Chem 2017, 3, 834-845
Carbohydrate-containing molecules are ubiquitous in nature and essential to sustaining lives. Selective modification of carbohydrates provides tremendous opportunities to interrogate the biological roles of this fundamental class of molecules. However, to functionalize a specific hydroxyl group out of numerous others in a carbohydrate scaffold has been a historical challenge. Here, we report a strategy that allows for the site-selective and site-divergent delivery of terminal propargyls to various monosaccharides, the basic units of carbohy- drates. This strategy is based on synergistic catalysis that combines copper and borinic acid catalysis. With a pair of antipodal ligands, this method can be tuned to propargylate either the equatorial or the axial hydroxyl with high selec- tivities. The generality of this transformation is manifest in the direct propargy- lation of complex, unprotected glycosylated natural products. Equipped with terminal alkyne groups, the products obtained by this method are primed for further manipulations, including copper-catalyzed azide-alkyne coupling chemistry.

Liqiang Wan, Lan Tian, Jie Liu, and Dawen Niu*.
Synlett 2017, 28(16), 2051-2056 (review)
The discovery and development of an Ir-catalyzed asymmet- ric umpolung allylation of imines is discussed here. This method pro- duces 1,4-disubstituted homoallylic amines, a class of compounds that are difficult to access by conventional methods. This reaction proceeds through a sequence involving an allylation and a 2-aza-Cope rearrange- ment event. The unique mechanistic feature of this reaction could be the reason for its broad substrate scope. The products of this reaction are useful intermediates for various bioactive and natural products. Be- sides its immediate synthetic utility, we expect this transformation to inspire the development of other umpolung functionalizations of imines and Ir-catalyzed asymmetric allylic substitution (AAS) reactions.

R-Z Li, H Tang, KR Yang, L-Q Wan, X Zhang, J Liu, Z Fu, and D Niu*.
Angewandte Chemie International Edition 2017, 56, 7213–7217
A copper/borinic acid dual catalytic reaction enabled the enantioselective propargylation of aliphatic polyols. Readily available reagents and catalysts were used in this transformation, which displayed good to excellent chemo‐ and stereoselectivity for a broad array of substrates. The method was also applicable to the desymmetrization of meso 1,2‐diols to furnish products with three stereogenic centers and a terminal alkyne group in one operation.

2016
J Liu, C-G Cao, H-B Sun, X Zhang, and D Niu*.
Journal of the American Chemical Society 2016, 138, 13103–13106
Here we report an iridium-catalyzed asymmetric umpolung allylation of imines as a general approach to prepare 1,4-disubstituted homoallylic amines, a fundamental class of compounds that are hitherto not straightforward to obtain. This transformation proceeds by a cascade involving an intermolecular regioselective allylation of 2-azaallyl anions and a following 2-aza-Cope rearrangement, utilizes easily available reagents and catalysts, tolerates a substantial scope of substrates, and readily leads to various enantioenriched, 1,4-disubstituted homoallylic primary amines.

M Zhan, R-Z Li, Z-D Mou, C-G Cao, J Liu*, Y Chen*, and D Niu*.
ACS Catal., 2016, 6, 3381–3386
Described here is an enantioselective approach of making chiral, β-substituted homoallylic organoboronic esters. In the presence of LiOtBu and a catalytic amount of silver salt, commercial bis[(pinacolato)boryl]methane partici- pated in the iridium-catalyzed asymmetric allylation reactions, delivered a “CH2B(pin)” group, and yielded the title compounds from allylic carbonates. The synthetic utility of the prepared chiral organoboronates was demonstrated by their conversion to other important classes of compounds.

Other Representative Publications
D Niu, PH Willoughby, BP Woods, B Baire, TR Hoye*
Nature 2013, 501 (7468), 531-534

D Niu, TR Hoye*
Nature Chemistry 2014, 6 (1), 34-40

D Niu, SL Buchwald*
Journal of the American Chemical Society 2015, 137 (30), 9716–9721
D Niu, TR Hoye*
Organic letters 2012, 14 (3), 828-831

